Abstract
Autologous gene therapy (GT) for beta-hemoglobinopathies has demonstrated encouraging early safety and efficacy using addition of a sickling-resistant globin gene to stem cells. Another therapeutic strategy for sickle cell disease (SCD) is erythroid-specific inactivation of BCL11A, which is a validated repressor of gamma globin expression (Sankaran et al. Science 2011). This approach has the distinct advantage of simultaneously inducing fetal hemoglobin (HbF) while coordinately decreasing sickle hemoglobin (HbS). Since hemoglobin (Hb) polymerization in sickle red cells is highly dependent on the intracellular concentration of HbS and is strongly inhibited by HbF, effective BCL11A repression should prevent the sickling phenotype within red cells. We have shown that erythroid-specific expression of microRNA-adapted shRNAs (shRNAmiR) targeting BCL11A effectively induces HbF in human erythroid cells derived from transduced HSCs, largely attenuating the hematologic effects of SCD in a murine model while avoiding negative effects in HSCs and B lymphocytes (Brendel et al. JCI 2016). Here we report the initial results of a pilot clinical study utilizing a shRNAmiR lentiviral vector (LVV) targeting BCL11A for autologous GT in SCD patients.
Transduction of hematopoietic cells with GMP lentiviral vector (BCH_BB-LCRshRNA(miR)) expressing the shRNAmiR for BCL11A in an erythroid-specific fashion showed no toxicity in engraftment and genotoxicity assays, efficient transduction rates of 80-95% of CD34+-derived erythroid colony forming cells from healthy donors and SCD patients, and >95% of transduced erythroid colonies demonstrating HbF levels of 50-95% of total Hb. Transduction at clinical scale with plerixafor mobilized CD34+ cells from three SCD donors yielded vector copies of 3.7 - 5.2/cell.
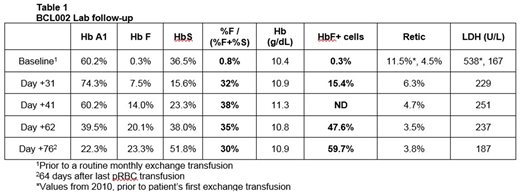
Patients with severe SCD were screened for eligibility according to an IND enabled, IRB-approved investigator-initiated protocol. The first cohort included patients ≥ 18 years old. After at least 3 months of protocol-required transfusions, autologous CD34+ cells were collected by plerixafor mobilization and apheresis, and then transduced under GMP conditions with the BCH_BB-LCRshRNA(miR) vector. As of July 28, 2018, 3 patients representing the adult cohort had undergone a total of 3 (n=2) or 4 (n=1) days of mobilization. Mean single-day apheresis yields were 3.2 (range 1.5 - 6.8) x 106 CD34+ cells/kg. No Grade 3 or 4 AEs were attributed to mobilization and collection, although one subject developed an incidentally-discovered line-associated atrial clot and pulmonary embolism. Transduced cell products for these 3 patients have cell doses of 3.3 - 6.7 x 106 CD34+ cells/kg, VCN of 3.3 - 5.1 copies per cell and >95% vector-positive CD34+-derived colonies. One subject (BCL002), who had been regularly transfused for 17 years, has undergone infusion of gene-modified cells after myeloablative busulfan conditioning and achieved neutrophil engraftment after 22 days. Post-infusion follow-up is 78 days. At the time of the last analysis 76 days after GT and 64 days after last RBC transfusion (Table 1) subject BCL002 had a sustained Hb of >10 g/dL and, compared to pre-GT, there was a notable absence of irreversibly sickled cells on peripheral smear and a persistently low absolute reticulocyte count consistent with markedly reduced hemolysis. Hb electrophoresis showed 23.3% HbF, 51.8% HbS and 22.3% HbA (from residual transfused cells) with a HbF/(HbF+HbS) ratio of 29.7%. At day 76, the number of F cells had risen to 59.7% with 12pg HbF/F cell. In flow-sorted immature erythroid cells γ-globin mRNA was >80% of total β-like globins in the graft-derived population and BCL11A protein was reduced by ~90%. Adverse events observed from the start of conditioning until latest follow-up were consistent with myeloablative conditioning, and there have been no product-related adverse events and no SCD-related complications.
These early results show: (1) feasibility of enrollment, cell procurement, and GMP manufacturing of gene modified CD34+ cells in 3 adult SCD patients; (2) the first proof of principle demonstrating shRNAmiR-based gene knockdown in humans, and (3) successful rapid induction of HbF in the first patient infused, with marked attenuation of hemolysis in the early phase of autologous reconstitution. Based on the trajectory of increasing HbF/(HbF+HbS), near full suppression of the SCD phenotype is expected.
Esrick:Bluebird Bio: Honoraria. Negre:bluebird bio: Other: Spouse employed by bluebird Bio. Dansereau:Bluebird Bio: Consultancy. Braunewell:Bluebird Bio: Employment, Equity Ownership. Christiansen:Bluebird Bio: Employment, Equity Ownership, Other: Salary. Nikiforow:Kite Pharma: Consultancy. Achebe:Luitpold Pharmaceutical: Consultancy; AMAG Pharmaceuticals, Inc.: Membership on an entity's Board of Directors or advisory committees; Syros pharmaceuticals: Consultancy. Negre:Bluebird Bio: Employment, Equity Ownership, Other: Salary. Heeney:Sancilio Pharmaceuticals: Consultancy, Research Funding; Ironwood: Research Funding; Novartis: Membership on an entity's Board of Directors or advisory committees; Vertex/Crisper: Other: Data Monitoring Committee; Pfizer: Research Funding; Astra Zeneca: Consultancy, Research Funding. Williams:Bluebird Bio: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal